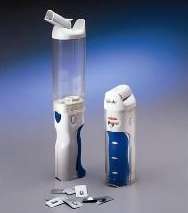
Patients with diabetes sometimes find it difficult to face the daily insulin injections.
Studies by Dr. Robert A. Gerber from Pfizer Global Research and Development in Groton, Mass. are showing that improvements in the lab tests for the diabetes marker hemoglobin A1C were similar for patients who received insulin inhalations to those patients who received the conventional injections.
The ease of use, comfort, as well as the overall satisfaction of inhalation as opposed to injection rated high. Long-term improvement in the control of blood sugar is maintained up to the 1 year follow-up.
In the future the patients may very well have the choice between inhalation and injection of insulin. Even though the 1 year follow-up results are in, longer follow-up studies are needed, before insulin shots become a thing of the past.
More info on diabetes: http://nethealthbook.com/hormones/diabetes/type-2-diabetes/
Based on Diabetes Care 2004; 27:1318-1323
Comment on Nov. 5, 2012: Pfizer marketed the inhalable insulin under the brand name “Exubera”. It was available in the US from Sept. 2006 onward after FDA approval. The inhalable insulin was proven to be as effective as the injectable insulin, but the cost of Exubera was prohibitive and Pfizer had to discontinue the production after October of 2007 as it was unlikely to be cost-effective, just 1 year and 1month after its initial release.
Last edited October 26, 2014