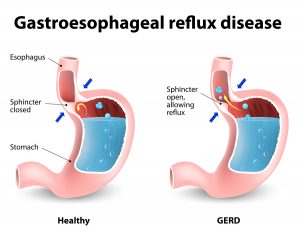
A study was recently published showing that death from heartburn drugs can come early, when compared to controls. The study was published in June 2017 in the online British Medical Journal Open. The researchers were located at the Washington University School of Medicine, Saint Louis, Missouri, USA.
They compared 349, 312 US veterans on proton pump inhibitors (PPI) to an equal amount of veterans on conventional H2 blockers. Over a follow-up period of 5.71 years there was an increased risk of death of 25% when patients took PPI drugs. No matter to what the researchers compared the PPI group to, there were always more deaths in the PPI group versus other control groups.
Causes of death
According to the senior author, Dr. Ziyad Al-Aly many deaths were due to kidney disease, dementia, fractures, pneumonia, Clostridium difficile infections and cardiovascular disease. Out of 500 patients who took the PPI drug there was one death within one year. But over the years the deaths increased. Dr. Al-Aly thinks that the PPI drug is interfering in some way with the genetic expression of some genes and suppressing others. These genetic differences may explain the early deaths.
As this was a retrospective study, it can only show an association of PPI drugs with earlier deaths, but this does not prove causation. It would require a prospective random study to prove causation.
Other studies regarding the risk of PPI drugs
An Icelandic study from May 2017 showed that there was a 30% increased risk of fractures in males and females following PPI drugs when observed over 10 years. Opiates had a risk of almost 50%, sedatives a 40% risk of increased fractures. Control groups of NSAIDs, statins and beta-blockers showed no increased fracture risk, nor did histamine H2-antagonists.
Side effects of PPI’s
An article from March 2017 is a critical review of the safety of PPI drugs. It notices that with long-term use there are adverse effects like fractures of the long bones, enteric infections and hypomagnesemia. PPI’s can increase the risk for heart attacks and can cause kidney disease and dementia. One of the problems is that gastroesophageal reflux usually dictates the long term use of anti acid drugs like PPI’s, but the longer patients are taking these drugs, the higher the death rate and side-effect rate. The physician should only use PPI drugs initially and after a few weeks switch to the less potent histamine H2-antagonists (like ranitidine).
Listeriosis as side effect of PPI’s
A Danish study from April 2017 noted an increased risk for listeriosis in patients who were on PPI drugs. Over 5 years there was a 2.81-fold higher risk of developing listeriosis in patients on PPI’s compared to a control group. If patients were on corticosteroids and a PPI the risk was even higher, namely 4.61-fold increase to develop listeriosis. In contrast, using histamine H2-antagonists had a risk of only 1.82-fold of developing listeriosis.
Poor prescribing habits for PPI’s
A Dec. 2016 study from Dublin, Ireland with patients older than 65 examined their PPI drug use. The comparison of data occurred between 1997 and for 2012. The researchers noted that the maximal PPI dose for long-term use was 0.8% of individuals in 1997 and 23.6% in 2012. The risk of prescribing high dose PPI drugs in 2012 was 6.3-fold in comparison to the risk in 1997. Examination of the health records showed that the indication for prescribing PPI drugs had no correlation with significant gastrointestinal bleeding risk factors. The study concluded that there was definitely room for improving prescribing habits.
Triple therapy
This January 2016 paper describes the standard treatment of H. pylori and gastric and duodenal ulcer treatment, which involves the triple therapy consisting of a PPI and two antibiotics. It pointed out that this treatment protocol “improves healing and prevents complications and recurrences”.
PPI’s causing risk for fractures
A paper from Leipzig, Germany dated July 2016 reviews the usage of PPIs. It mentions that there has been a significant increase of prescriptions in the past 25 years. Patients on PPI’ are at a greater risk for fractures. There is also a risk of low B12 levels from malabsorption of B12. The physician should check this from time to time, and if necessary give B12 injections.
Clostridium difficile infections
A Canadian study from May 2015 found that Clostridium difficile infections (CDI) were linked to chronic antibiotic use or to prolonged use of high doses of PPI drugs. There was a 1.5-fold risk of recurrent CDI in patients older than 75 years who were taking PPI drugs continuously. There was a 1.3-fold recurrence of CDI after antibiotic re-exposure.
Alternative remedies for heartburn
- Dr. Weil recommends the use of deglycyrrhizinated licorice (DGL) for heartburn or early ulcers.
- Here is a clinical study with 56 patients with duodenal and gastric ulcers that was published in 1968. Both radiographic evidence as well as clinical findings showed that the ulcers healed and that stomach spasms subsided with DGL treatment. Nobody knew at that time that DGL had antibacterial effects and that often chronic heartburn, stomach and duodenal ulcers can be due to H. pylori infections that are simultaneously present.
- A December 2016 study showed that probiotics could be a valuable adjunct in triple therapy for H. pylori infection. The study also points out that H. pylori is present in about 50% of the world’s population.
Antibacterial effects of DGL
- A paper of December of 2012 shows that an important tooth decay bacterium responds to DGL.
- In a 1989 study 20 patients with aphthous mouth ulcers were followed. DGL mouthwash led to a 50 to 75% improvement in 15 patients within one day of treatment and by the 3rd day there was complete resolution.
- Here is a suggestion of a four-step approach against H. pylori.
- DGL has been shown to be useful in gut regeneration in patients with Clostridium difficile infection.
Discussion
I started with a review of a recent paper that pointed out the side effects of PPI drugs. PPI’s are common medications for acid reflux disease, stomach and duodenal ulcers, either alone or as part of the triple therapy. Chronic infection of H. pylori is often the cause of these problems. I reviewed the literature surrounding deglycyrrhizinated licorice (DGL), a natural antacid remedy. It turns out that DGL can be quite useful either as a parallel treatment or instead of the triple therapy.
The problem over the past 25 years is that physicians have been treating acid problems with higher and higher doses of PPI’s. They are also using ASA prophylaxis against heart attacks and strokes more often. This has caused gastric erosions that are bleeding, which in turn caused physicians to prescribe more PPI’s. The side effects of PPI’s belongs to the iatrogenic (doctor- induced) diseases. This is an artificial disease that occurs from the side effects of overprescribed medicine. PPI’s are a very useful short-term anti-acid medication. However, do not use this medication for more than 4 to 8 weeks. But as patients receive years and years of this medication, serious problems like heart attacks, fractures, kidney disease, dementia, and pneumonia as well as Clostridium difficile infections become the consequence. Overall there was an increase of the death rate of 25%.
It sounds quite reasonable that doctors should return to a more conservative approach as the FDA has suggested. This includes alternative natural methods including DGL and probiotics.
Conclusion
A recent study from the online British Medical Journal Open has pointed out a high death rate among long-term proton pump inhibitor (PPI) drug users. The se drugs are used to suppress acid formation in the stomach. They are helpful, if there are significant gastrointestinal bleeding risk factors present. But prolonged use of PPIs causes severe side effects as described, including a chronic persistent Clostridium difficile infection (CDI) of the gut that can become resistant to antibiotic therapy. In cases of recurrent CDI one important step is to discontinue PPIs. The physician should consider switching to one of the conventional histamine H2-antagonist drugs (like ranitidine). Overusing PPIs in an older population is not responsible, as this leads to disease that is caused by a physician! There is no need for this to happen.
Avoiding toxic drug levels of PPI’s
The prescribing physician has to exercise caution and restraint and the patients, and their loved ones need to be aware of multidrug interactions. PPIs belong to the drugs that are eliminated in the liver through the cytochrome P450 enzyme system (CYP2C19). But this enzyme system interfering with the drug elimination process may also eliminate other drugs taken by the patient. The end results can be toxic drug levels of PPIs. It can potentiate the side effects and become responsible for the 25% increased risk of death when the patient takes PPI drugs chronically. Even though PPIs are the newer medication, newer does not always mean better.