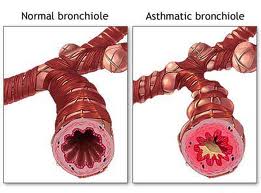
Patients with severe asthma will have a new treatment option. A new medication under the name omalizumab has been studied in clinical trials by Dr. Jean Bousquet of Arnaud de Villeneuve Hospital in Montpellier, France.
In the study it was also determined which patients would benefit most of the new drug. The results are very encouraging: those patients who had the least success with other asthma medications had the best response. However, it is not an instant response. 67 % of the patients showed a good response after 4 weeks of treatment. 87 % of patients showed a response only after having been on the medication for 12 weeks. As a result of this trial it was determined, that patients with asthma should be treated with omalizumab for at least 12 weeks. FDA approval for difficult to manage cases of allergic asthma came in June of 2003 (trade name Xolair, manufacturer: Genentech, Inc). On the other hand, patience is also of essence: if the medication is only administered for a month, a lot of patients with difficult to treat asthma will miss out on the beneficial effects, as opposed to those who persist and reap significant improvement after 12 weeks. One of the downsides of the medicine is the possibility of anaphylactic reactions.
Reference: National Review of Medicine, May 15, 2004, pg. 27
More information about asthma: http://nethealthbook.com/lung-disease/asthma-introduction/
Here is a NEJM article (case study involving omalizumab)
Last edited Oct. 26, 2014