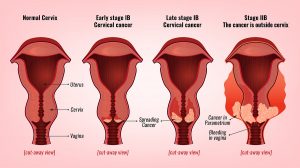
HPV testing for cervical cancer is more sensitive than the traditional Pap test. For years physicians recommended the traditional Pap test once a year to prevent cancer of the cervix. But a few years ago a new cervical cancer screening test, namely the HPV test made the news. It stems from the observation that cancer of the cervix develops in 99.7% of women who test positive for the HPV virus. There are many types of HPV, here we are interested in the few subtypes that produce cancer (carcinogenic HPV virus).
Transmission of the HPV virus between men and women
The human papilloma virus transmits from males to females through bisexual contact. The problem starts when he develops HPV lesions on his penis. Without him wearing a condom, the contact with her cervix during sex can transmit HPV to her cervix. Both partners are not aware of the transmission of that virus, as it does not cause any symptoms. HPV invades the superficial skin layer of the cervix in the woman. In the man HPV will invade the skin of the glans of the penis. After certain incubation time it causes transformation into cervical cancer in the woman. Strangely enough it does not cause cancer in the male. However, in both sexes HPV virus is in the mucous membranes and can contaminate the other sex’s genital.
A recent study comes from UBC Vancouver, British Columbia, which compared the Pap test with HPV testing.
Details of the Vancouver study on HPV testing for cervical cancer
On July 3, 2018 this study appeared in the medical journal JAMA.
19,009 women were part of this randomized clinical trial. With HPV testing only 2.3 cases per 1000 women of early cervical cancers were present four years later. Using the traditional Pap test this figure was 5.5 cases per 1000 women after 4 years. 224 clinicians participated in this study. Women were recruited for this study from January 2008 to May 2012. Follow-up took place till Dec. 2016. The participating women in this study were 25 to 65 years of age.
In 2017 in the US there were still 12,820 women in the United States who got cancer of the cervix. Approximately 4210 are dying from this disease every year. Many women do not like to take the Pap test or the HPV testing. There are compliance problems with either one of these tests.
Significance of this trial regarding HPV testing for cervical cancer
The newer HPV testing was superior to the regular Pap test. The HPV test was more sensitive and resulted in much lower cancer rates after 4 years of follow-up. Every woman would have an HPV test every 4 years. In this case we likely would see cervical cancer go to the bottom of cancers that kill women. The reason for that is that HPV testing and colposcopy pick up cancers much earlier. This leads to a more effective treatment of cervical cancer. After 4 years much less cancer of the cervix was found when the researchers tested again using HPV testing.
Implications of HPV testing for cervical cancer
In third world countries
Many 3rd world countries do only the HPV testing. At the time when this decision was made, it was unknown that they had actually chosen the better method to test for cancer of the cervix. Now this trial reassures all the health care providers in 3rd world countries that they should continue with the program, and they only have to do the test every 4 years, not every 2 years, which makes it even more cost effective.
Implications for the US
In the US so far the recommendation was to do both the regular Pap test and the HPV test simultaneously. This trial, however, says that this is not necessary. It would be better to use the more sensitive HPV test and abandon the more expensive and less sensitive PAP test. In 2012 a taskforce recommended to do the Pap smear in women age 21 to 65 every 3 years. The taskforce further recommended to women age 30 to 65 that they screen with a combo of cytology and HPV testing every 5 years. The lead investigator, Dr. Ogilvie said: “Offering women HPV [testing] for cervical cancer screening detects more precancerous lesions earlier, and also a negative HPV test offers more assurance that women will not develop precancerous conditions in the next four years,” she said. “This can mean that women may need less frequent screening but have more accurate results.”
What other doctors are saying about HPV testing for cervical cancer
Comments by Dr. Kathleen Schmeler
Dr. Kathleen Schmeler said that the study was “well-designed” and provided a much-needed comparison of Pap versus HPV testing. She is a gynecologic oncologist and at The University of Texas MD Anderson Cancer Center. She was part of the new research. Dr. Schmeler added: “The bottom line is that this could really potentially simplify how we screen women and have it be more effective and not quite as complicated and burdensome — and opens the door for doing just HPV testing, which is actually what’s currently recommended by the World Health Organization for countries that don’t have Pap testing capabilities,”
Comments by Dr. Stewart Massad
Dr. L. Stewart Massad Jr. is a professor of obstetrics and gynecology in the division of gynecologic oncology at Washington University School of Medicine. He wrote an editorial to the study in the JAMA. He wrote: “What will replace the Pap test? In 2012, the American Cancer Society endorsed co-testing with cervical cytology testing and HPV testing at 5-year intervals as the preferred strategy for screening women 30 to 65 years of age because this approach combines the sensitivity of HPV testing with the familiarity of traditional Pap testing,” He then went on to say: “However, the addition of cervical cytology testing adds little to the accuracy of HPV testing while increasing cost and false-positive results. In 2018, organizations that develop cancer screening guidelines are wrestling with whether to recommend replacing co-testing with primary HPV testing as the optimal screening strategy.”
Future dilemma
In view of all those comments the regulatory agents will have to come up with solutions for what is in the best interest of women for testing for cervical cancer.
Conclusion
A large randomized clinical trial in Vancouver, BC, Canada has compared screening methods for cancer of the cervix in women. Half of the subjects underwent screening by the newer HPV tests that checks for the presence of HPV virus. The other half received conventional screening by the Pap test (a cytological screening test.) The result was that the HPV test was more sensitive and resulted in less early cancer tests 4 years down the road. With the conventional Pap test there were more than double the amount of abnormal cells present 4 years down the road, which makes the Pap test less safe compared to the HPV test.
It appears from this trial that the Pap test is no longer a choice, except for colposcopy procedures that take care of early cervical cancers. But for screening in general HPV testing every 4 years is all what every women needs for her protection.
Related topics:
- Cancer rates increased in women.
- Catch cancer early.
- HPV testing was described in this blog in 2013: Low cost cervical cancer screening.