A new publication found that repairing epigenetic defects can slow down aging. This is a newer concept, which is gaining momentum in the longevity research world. David Sinclair is a professor of genetics at the Blavatnik Institute at Harvard Medical School. He is also co-director of the Paul F. Glenn Center for Biology of Aging Research.
Here is the summary of how epigenetic factors can slow down aging:
- Double-stranded DNA breaks can erode the epigenetic landscape
- Loss of epigenetic information accelerates the hallmarks of aging
- Epigenetic reprogramming changes aging into more youthful appearance
- Researchers can manipulate the epigenome, thus driving aging forward and backward
Professor Sinclair said that the conventional thinking about aging is no longer sustainable. The old theory was that genetic mutations undermine our DNA, creating a junkyard of damaged cellular tissue that leads to deterioration, disease and death. Instead, Dr. Sinclair said: “We believe it’s a loss of information – a loss in the cell’s ability to read its original DNA so it forgets how to function – in much the same way an old computer may develop corrupted software. I call it the information theory of aging.”
Here is what Dr. Sinclair and his team have established
- DNA in the cells function like the body’s hardware. The epigenome is the software.
- Proteins and chemicals make up the epigenes that sit “like freckles on each gene”. It is the epigenome that turns genes on and off. Pollution, environmental toxins, human behaviors such as smoking, eating an inflammatory diet or suffering a chronic lack of sleep will all trigger a change in the epigenetic information. Dr. Sinclair added: “And just like a computer, the cellular process becomes corrupted as more DNA is broken or damaged.”
- “The cell panics, and proteins that normally would control the genes get distracted by having to go and repair the DNA,” he explained. “Then they don’t all find their way back to where they started, so over time it’s like a Ping-Pong match, where the balls end up all over the floor.”
- “The astonishing finding is that there’s a backup copy of the software in the body that you can reset,” Sinclair said. “We’re showing why that software gets corrupted and how we can reboot the system by tapping into a reset switch that restores the cell’s ability to read the genome correctly again, as if it was young.”
- Sinclair went on to say: “It doesn’t matter if the body is 50 or 75, healthy or affected by disease. Once that process has been triggered, the body will then remember how to regenerate and will be young again, even if you’re already old and have an illness. Now, what that software is, we don’t know yet. At this point, we just know that we can flip the switch.”
History of longevity research
Dr. Sinclair started his research into longevity as a graduate student by working with yeast cells. He studied the genes that controlled aging in yeast. After he identified the aging gene, he also could determine the same gene in mice. He developed ICE, short for “inducible changes to the epigenome”, basically temporary, fast-healing cuts. They mimic the aging process due to exposure to pollution and sunlight. Mice treated with the ICE method for one year looked like regular mice would look after two years. The Sinclair team of researchers were able to age tissues in the brain, eyes, muscle, skin and kidneys of mice using the ICE method.
Mice turning younger again
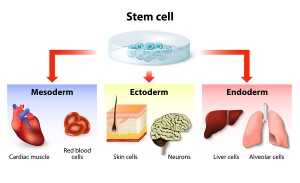
But what is more important, they also showed that these aged mice could turn younger again. Dr. Sinclair’s researchers took human adult skin cells, reprogrammed them to behave like embryonic or pluripotent stem cells and injected them into the older mice. To their surprise this triggered the test animals to become younger again. Dr. Sinclair said that fortunately they went back to an age between 50% and 75% of the normal life span. None of the animals developed cancer. The researchers injected the cocktail into damaged retinal ganglion cells at the back of the eyes of blind mice. Then they activated the rejuvenation process of the retinal ganglion cells by feeding the mice antibiotics. Surprisingly, the mice regained most of their eyesight. In a similar way the researchers could rejuvenate aged muscle cells, skin cells and kidney cells of mice.
Human longevity
We are not at the point yet where human research similar to mouse research is being done. But Dr. Sinclair is convinced that more human data will be on the way. He pointed out that telomere tests of leukocytes in blood tests of individuals with healthy lifestyles already show that they have a significantly lower biological age compared to their chronological age. His advice for longevity is this:
- Focus on plants for food and eat less often
- Get sufficient sleep
- Exercise regularly and get short-winded for 10 minutes at least three times a week
- Regular exercise will also help you to maintain your muscle mass
- Don’t get upset about unimportant things. In other words, manage your stress
- Have a good social support group
Other ways to reduce your biological age
I attended the 22nd Annual World Congress on Anti-Aging Medicine In Las Vegas (Dec. 10-14, 2014) that dealt with telomere length and how nutrition can positively influence what our genes express. This ultimately determines how long we live. Dr. Al Sears gave one of the talks at the conference. He pointed out that shortened telomeres are causing cells to behave like old cells. In the lab we can lengthen telomeres. Telomerase activated animals regrew their brains! In the human situation the goal is to find ways to preserve the length of our telomeres in all our key organs. Dr. Sears said a patient with short telomeres who starts telomerase stimulating supplements, develops longer telomeres in leukocytes within one month of commencing the supplementation.
The following supplements lengthen telomeres
Dr. Sears pointed out that the following supplements lengthen telomeres in humans.
- Acetyl-L-carnitine and resveratrol are two substances that reliably elongate telomeres.
- Vitamin C will significantly delay shortening of telomeres resulting in delayed aging.
- In addition, researchers showed recently that vitamin C stimulates telomerase activity in certain stem cells.
- The herbal Silymarin extract increases telomerase activity threefold.
- N-acetyl cysteine is a building block for glutathione, a powerful antioxidant. In addition, it has been shown to turn on the human telomerase gene.
- Other telomerase stimulators are green tea extract, ginkgo biloba, gamma tocotrienol (one of the components of the vitamin E group), vitamin D3 and folic acid.
The following link lists Dr. Sears’ recommended daily doses of these telomerase stimulating supplements.
Conclusion
Epigenetic defects appear to be more important than genetic abnormalities. The problem is that epigenetic changes through pollution or sun exposure can switch off genetic switches. This makes us age much faster than healthy controls. In this review I described mouse experiments that Professor David Sinclair did. He is professor of genetics in the Blavatnik Institute at Harvard Medical School, Boston, MA. He demonstrated in mice that he could age them faster with the ICE method, short for “inducible changes to the epigenome”, basically temporary, fast-healing cuts. They mimic the aging process due to exposure to pollution and sunlight. He also showed that these aged mice could turn younger again.
Rejuvenation of older mice
Dr. Sinclair’s researchers took human adult skin cells that have been reprogrammed to behave like embryonic or pluripotent stem cells and injected them into the older mice. To their surprise this triggered them to become younger animals again. None of them developed cancer and they turned back to 50% to 75% of their original age. There is no application to humans yet, but Dr. Sinclair’s team is planning to address this issue next.