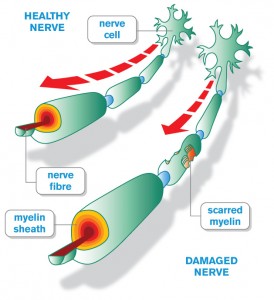
MS, the debilitating and at times fatal disease which affects about 50,000 Canadians continues to be a puzzle to medical researchers. New findings are shedding new light on this illness and may help to unravel its complexities and bring more effective treatment to patients.
Dr.Brenda Banwell from the Department of Pediatrics and the Pediatric MS Clinic at The Hospital For Sick Children in Toronto found that 83 % of children with a diagnosis of MS showed evidence of a previous Epstein-Barr virus infection. (Healthy controls only showed a rate of 42 %). No differences were found for other viruses (like herpes, parvovirus, chicken pox). Researchers have yet to determine, whether there is a link between Epstein-Barr virus infections and MS, or whether MS patients are more susceptible to Epstein-Barr infections.
With regard to MS treatment amazing improvement has been demonstrated on MS patients who were treated with the cholesterol-lowering drug simvastatin. A reduction of MS induced brain lesions by 44 % was achieved in patients treated with the drug, and animal experiments show similar results. Researchers are cautioning MS patients that more investigations will be needed, till this treatment will become a new standard in the treatment of MS.
Link to more information on multiple sclerosis.
Reference: Parkhurst Exchange, Vol.12, Nr.8, August 2004,page26
Last edited December 8, 2012